The tube is bent into a "U" shape and mounted on an inexpensive piezoelectric speaker. The resonance frequency of the tube is detected by a photointerrupter, amplified, and fed into the speaker; the resulting feedback circuit keeps the glass tube vibrating at its resonance frequency. A peristaltic pump and computer are used to flow samples through the tube and record the resonance frequency of the tube. Detail of the glass tube shows the path followed by a sample inside the tube as it flows into the sensor ("a"), passes the tip of the sensor ("b"), and exits the sensor ("c"). Resonance frequency of the vibrating tube vs. time as a 770 μm diameter glass bead is passed back and forth through the tube eighteen times.
Each passage of the bead through the tube results in a momentary decrease in the tube's resonance frequency; this is recorded as a downward peak in the resonance frequency . Each point on the peak (baseline "a," tip "b," and baseline "c") corresponds with the bead's location in above. The height of this peak is used to determine the buoyant mass of the bead . Histograms showing the buoyant mass of another bead weighed thousands of times in two different fluid densities. In deionized water (density 1.000 g/mL) the bead has an average buoyant mass of 3.69 ±0.12 μg, and in a sodium chloride solution (density 1.046 g/mL) the bead has an average buoyant mass of −4.42 ±0.16 μg.
The widths of these distributions—120 and 160 nanograms—provide an estimate of the resolution of our mass measurements. When a sample passes through the vibrating tube sensor, its buoyant mass is recorded as a brief peak in the plot of resonance frequency vs. time (e.g., Fig 1C and 1D). Once a peak is located, the height of the peak can be measured and converted to a corresponding buoyant mass value using the sensor's point mass calibration described below. Alternatively, a custom Python program can be used to fit the raw frequency measurements to an analytical equation of expected peak shape derived from Dohn et al. The resulting buoyant mass measurements were recorded and processed using a moving window average filter with a window size of five data points to slightly reduce noise in the plots of buoyant mass vs. time. Additional details about signal processing are provided in S6 Fig.
The four proof-of-concept samples studied here—microbeads, embryos, seeds, and biomaterials—are representative of a wide range of samples that may be analyzed in fluid using vibrating glass tube sensors. Our technique is very versatile because all objects have fundamental physical properties like mass. Consequently, our mass sensor can be applied to problems as diverse as screening toxic substances, understanding the growth of plants, measuring the degradation of biomaterials, and many others. And unlike imaging-based measurements of size, our mass sensor is insensitive to the shape of the object. Finally, the automation, portability, and low cost of this technique make vibrating glass tubes particularly well suited for applications in the field or in resource-limited settings. To measure the density of a sample of material, both the mass and volume of the sample must be determined.
For both solids and liquids, a balance can be used to measure mass; however, methods for determining volume are different for solids and liquids. As liquids can flow and take the shapes of their containers, glassware such as a graduated cylinder or volumetric flask can be used to measure the volume of a liquid. The volume of an irregularly-shaped solid can be measured by submersion in a liquid — the difference in volume caused by addition of the solid is equal to the volume of the solid. In this work, we demonstrate a simple and inexpensive sensor capable of weighing microgram-sized objects in fluid. Like the SMR, this sensor uses a change in resonance frequency to weigh an object in fluid with high precision. But unlike the SMR, this sensor can weigh samples with a large range of sizes and is extremely simple to fabricate.
Our sensor consists of a short length of glass tubing bent into a "U" shape and attached to an inexpensive speaker that vibrates the glass tubing at its resonance frequency. The resulting sensor shown in Fig 1 costs about US $12 in materials and can be made in under 10 minutes. Additionally, by weighing samples in fluids of different densities, we can also use our sensor to measure the volume and density of samples in fluid. Before using a vibrating tube to weigh a sample, the tube must first be calibrated.
We calibrated the sensor shown in Fig 1A using a glass bead of known size and density in a fluid of known density . Fig 1C shows eighteen downward peaks in the resonance frequency of the tube as the bead is pumped back and forth eighteen times through the tube. The height of each peak (72 millihertz; Fig 1D) is proportional to the buoyant mass of the bead .
Our homemade vibrating tube mass sensors typically have quality factors around 500, which is comparable to that of tuning forks and high enough for precise measurement of the tube's resonance frequency . Most solid substances are irregularly shaped, which complicates volume determination. It is inaccurate, for example, to determine the volume of a powder by measuring its dimensions. Instead of directly measuring dimensions or using glassware like a volumetric flask, it is necessary to make use of a liquid displacement method to measure the volume of an irregularly shaped solid. A graduated cylinder containing a known volume of liquid is tared. The solid is added to the cylinder, and the total mass is weighed again to determine the mass of the solid.
Addition of the solid causes an upward displacement of the liquid, resulting in a new volume reading. The volume of the solid is equal to the change in volume due to liquid displacement (i.e., the difference in liquid volume before and after adding solid). Where mo is the absolute mass of the object, ρo is the density of the object, and ρf is the density of the fluid filling the channel. Stated in words, an object's buoyant mass is equal to its real mass minus the mass of an equivalent-volume amount of fluid. If the object's density is less than the fluid's density, then the object has a negative buoyant mass and its passage through the tube will result in a momentary increase in the tube's resonance frequency . Finally, if the object's density equals the fluid's density, then the object will have zero buoyant mass and its passage through the tube will have no effect on the resonance frequency of the tube .
Thus, the orientation of the vibrating tube with respect to gravity has no effect on its measurements. To determine the density of an irregular solid in pellet form, add approximately 40 mL of water to a clean and dry 100-mL graduated cylinder. Place the cylinder on an analytical balance and tare.
Add approximately 10 pellets, and record the new volume after the addition. The mass is only the pellets, as the rest have been tared. Make at least two additional sets of mass and volume measurements to calculate an average value of the density. The density for zinc was measured for three different samples. Note that, since the measurements were made in a graduated cylinder, which is less precise than a volumetric flask, the density has lower degree of precision. Measurements of an object's fundamental physical properties like mass, volume, and density can offer valuable insights into the composition and state of the object.
However, many important biological samples reside in a liquid environment where it is difficult to accurately measure their physical properties. Of a substance is the ratio of the mass of a sample of the substance to its volume. The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, however, this as an inconvenient unit, and we often use grams per cubic centimeter (g/cm3) for the densities of solids and liquids, and grams per liter (g/L) for gases. Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 to 19 g/cm3 . Table \(\PageIndex\) shows the densities of some common substances.
This study aims to design a mechanism with which the density of any solid or liquid can be determined without measuring its mass and volume in order to help students comprehend the concept of density more easily. The solidensimeter comprises of two scaled and nested glass containers and sufficient water. Using this method, the density of any solids or liquids can be determined using a simple mathematical ratio. At the end of the process a mechanism that helps students to comprehend the density topic more easily was designed. The system is easy-to-design, uses low-cost equipment and enables one to determine the density of any solid or liquid without measuring its mass and volume.
Raw resonance frequency data from repeated measurements of a single polyethylene microbead in water (from the right-hand distribution of measurements in Fig 1E). After zooming in to the filtered data , peaks corresponding to individual measurements of the microbead are visible. Zooming in further on one pair of peaks shows the ∼2 millihertz height of these peaks (corresponding to a buoyant mass of ∼3.7 μg for this microbead). The peaks come in pairs because the particular vibrating tube sensor used for this measurement had a tuning-fork shape with two vibrating "U"-shaped lobes . Volume is the amount of space an object occupies while density is the mass of an object per unit volume.
You need to know the volume of an object before you can calculate its density. Calculating volume for regular objects can be done with a simple formula determined by the shape of the object. Common units for volume are cubic centimeters , cubic meters , cubic inches , and cubic feet .
Once you have the volume, density is one more simple calculation away. Common units for density are grams per cubic centimeter (g/cm3) or grams per milliliter (g/mL). Table 1 lists results for the determination of the density of ethanol using a 50-mL volumetric flask. Densities were calculated by dividing the measured mass by 50.0 mL.
The mean measured density was 0.789 ± 0.001 g/mL. Table 2 lists results for the determination of the density of a sample of zinc metal using a 100-mL graduated cylinder and the liquid displacement method. Note that the measured densities are constant for both substances. Table 2, in particular, demonstrates that density is independent of the amount of substance studied. This demonstration illustrates the methods for measuring the density of solids and liquids. Using a volumetric flask and an analytical balance, the density of ethanol can be determined.
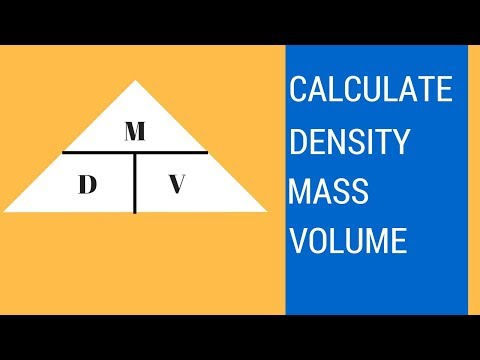
Using a graduated cylinder, analytical balance, and water as the displaced liquid, the density of zinc metal can be determined. Volume is an amount of space, in three dimensions, that a sample of matter occupies. The number and the phase of the molecules in the sample primarily determine the volume of a substance. Volume will be measured in many ways in this course, but the units are usually milliliters or cubic centimeters . Methods for determining or delivering precise volumes include volumetric pipets and pycnometers; less precise methods include burets, graduated cylinders, and graduated pipets. Ρ is the object's density m is the object's total mass V is the object's total volume Under specified conditions of temperature and pressure, the density of a fluid is defined as described above.
However, the density of a solid material can be defined in several ways. Porous or granular materials have a density of the solid material, as well as a bulk density, which can be variable. For example, if you gently fill a container with sand, and divide the mass of sand by the container volume you get a value termed loose bulk density. If you took this same container and tapped on it repeatedly, allowing the sand to settle and pack together, and then calculate the results, you get a value termed tapped or packed bulk density. Tapped bulk density is always greater than or equal to loose bulk density. In both types of bulk density, some of the volume is taken up by the spaces between the grains of sand.
The density of the sand grains, exclusive of the air between the grains, will be higher than the bulk density. The density of a substance can be used to define the substance.Water is unusual because when water freezes, its solid form is less dense than liquid water, and thus floats on top of liquid water. A little-known method of measuring the volume of small objects based on Archimedes principle is described, which involves suspending an object in a water-filled container placed on electronic scales. The suspension technique is a variation on the hydrostatic weighing technique used for measuring volume.
The accuracy and precision of the three methods was compared using 10 accurately machined PVC cylinders ranging in volume from 1.5 to 15.7 ml. The mean difference between the actual and measured volumes was 3.3 +/- 7.3%, -1.6 +/- 7.2% and 0.03 +/- 0.45%, for the level, overflow and suspension methods respectively. Each measurement was repeated twice to obtain the reproducibility of the three displacement techniques. The reproducibility was –1.7 +/- 8.5%, 0.09 +/- 3% and –0.04 +/- 0.43% for the level, overflow and suspension techniques respectively.
The results show that the suspension technique is more accurate and precise than the traditional water displacement methods and is more accurate than measuring volume using Vernier calliper measurements. A simple method, which is based on the principle of moment of forces only, is described for the determination of the density of liquids without measuring the mass and volume. At first, an empty test tube and a solid substance, which are hung on each side of a metre rule, are balanced and the moment arm of the test tube is measured. Keeping the solid substance in the same position, the test tube is filled with a liquid of known density up to a mark and then balanced. Then the test tube is filled with the unknown liquid up to that mark and after balancing the moment arm is measured. By measuring these three moment arms and using the density of the known liquid, the density of the unknown liquid is determined.
Finally, to demonstrate our technique using a biologically-relevant sample other than an organism, we used vibrating glass tubes to precisely measure the degradation rates of biodegradable materials. For many applications in medical implants, it is desirable to have materials with known degradation rates. For example, a screw for repairing a broken bone might remain intact until the bone heals and then dissolve away. However, measuring the degradation rates of slow-degrading materials is a time-consuming and labor-intensive process.
This process slows the development of new biomaterials and introduces the potential for human error. As an object passes through the vibrating tube sensor, the shape of each resulting peak in the tube's resonance frequency is a function of the vibrational mode and amplitude of the tube. In this work, the tubes are vibrating at their primary vibrational mode, meaning that the amplitude of vibration is highest at the tip (the bottom of the glass "U") and lowest at the base (the top of the "U"). As the particle leaves the tube (point "c" in Fig 1B) the tube's vibrational amplitude in this region decreases again, so the resonance frequency of the tube returns to baseline (point "c" in Fig 1D). General mathematical expressions for predicting this peak shape for any vibrational mode were derived by Dohn et al.
Magnesium ribbon with a thickness of 250 μm (98% pure; MiniScience Inc., Clifton, NJ) was used as a model biomaterial in our degradation rate measurement studies. Roughly 1 mm sized pieces of magnesium were cut from the ribbon. The samples were polished before measurement using 600, 800, and 1200 grit silicon carbide abrasive papers to remove the native oxide layer. Flow through the sensor was controlled using the servomotor as described previously. The resulting buoyant mass measurements are shown in Fig 4.
Density is a measure of the mass per unit volume of a substance. Think of it as the amount of mass that a substance packs into the space it takes up. You can calculate the density of any substance by dividing its mass by its volume. Density is usually measured in kilograms per cubic meter (kg/m3), but for the purposes of this experiment, we'll be measuring it in smaller, more "measuring cup-friendly" units of grams per milliliter (g/ml). By convention, the volume of liquids and gases is often expressed in units of liters or milliliters, measured with glassware. The dimensions of regularly shaped solids can be measured directly with rulers or calipers, which have linear units, giving volumes in units such as cubic centimeters.
One milliliter is equivalent to one cubic centimeter. The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Increasing the pressure on an object decreases the volume of the object and thus increases its density.
Increasing the temperature of a substance decreases its density by increasing its volume. Seeds of iceland poppy , oregano , and foxglove were obtained from Ferry-Morse and measured every 10 seconds in water until active germination was observed using the servomotor reservoir lifter described above. This estimate of single-seed mass was used along with the seed buoyant mass measurements to calculate the estimated density of each seed during imbibition and germination (Fig 3G–3I). However, there are many important samples that are too large to be weighed in the microfluidic SMR and too small to be weighed on a conventional balance. For example, fish embryos are used in a variety of developmental biology and toxicology applications . If the weight of an embryo could be monitored as it grows or reacts to stimuli, these measurements could offer new insights into developmental biology and toxicology.
However, millimeter-sized zebrafish embryos are far too large for the micron-scale channels in the SMR, and the aquatic environment of the embryos complicates weighing them using a traditional balance. But accurately predicting the degradation rates of these materials in the fluidic environment of the body is challenging. Keeping the solid substance in the same position, the test tube ...
The density at all points of a homogeneous object equals its total mass divided by its total volume. The mass is normally measured with a scale or balance; the volume may be measured directly or by the displacement of a fluid. To determine the density of a liquid or a gas, a hydrometer, a dasymeter or a Coriolis flow meter may be used, respectively. Similarly, hydrostatic weighing uses the displacement of water due to a submerged object to determine the density of the object.






















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.